The research is carried out in cooperation with the Institute of Chemistry, Technology and Metallurgy, University of Belgrade.
Pollution of watercourses with organic materials (pesticides, dyes), toxic elements, such as arsenic and chromium, as well as pharmaceutical residues is becoming alarming. These pollutants significantly affect the environment, and in humans lead to endocrine, hormonal and genetic disorders and they cause severe illnesses including cancer. The problem of water pollutants is present in all countries of the world. Various physical, chemical and biological treatments are used for water purification. All of them are often insufficiently efficient or expensive. With the development of nanoscience and nanotechnology, a new field of research is opened that involves the detection and removal of water pollutants using new procedures based on the use of magnetic nanomaterials.
The goals of our research are to design and synthesize materials that would have high adsorption power for toxic elements such as arsenic and chromium compounds and/or would be used for modification of working electrodes for the detection of toxic elements and organic pollutants, as well as the degradation of organic dyes by the electrochemical procedure. Iron oxide nanoparticles (IONP) with different morphologies (spherical, cubic, flower-like and needles) were employed for surface decoration of the most common carbon-based materials (graphene, graphene oxide, carboxylated graphene, graphene nanoribbons, graphene nanoplatelets, single- and multi-wall carbon nanotubes), and the nanocomposites formed were deposited on the electrode surface. Removal of RB52 and RB5 with these electrodes was about twice as effective in comparison with bare SPCE, reducing the time needed for complete degradation to only 30 min. The effects of morphology, size, type of carbon-based material used for nanocomposite formation on the degradation efficiency were examined.
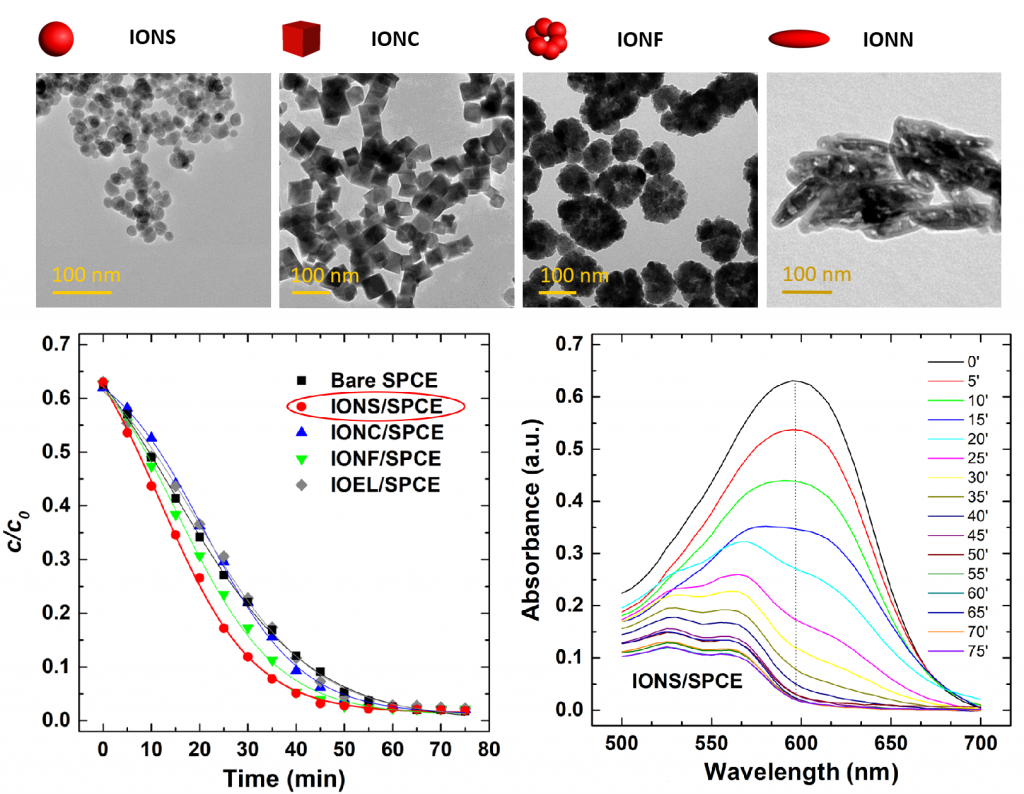
(M. Ognjanović et al., Tailoring IONP shape and designing nanocomposite IONS@GN toward modification of SPCE to enhance electrochemical degradation of organic dye, Mat. Res. Express, 7 (2020) 015509).
Effects on the morphology of partial substitution of Fe3+ by Y3+ in magnetite (Fe3O4), and inorganic arsenic species adsorption efficiency of formed Fe3-xYxO4 nanoparticles, were investigated. With the increase of yttrium content (x value), increase both morphological inhomogeneity of the sample, and a fraction of spinel nanorods as compared to spinel pseudospherical particles. The Fe3-xYxO4 affinity of adsorption toward inorganic arsenic species As(III) (arsenite, AsO33−) and As(V) (arsenate, AsO43−) were determined. An increase of Y3+ concentration related to changes in sample morphology was followed by a decrease of As(III) removal efficiency and vice versa for As(V). The increase in the Y3+ content, in addition to increasing the adsorption capacity As(V), significantly expanded the optimum pH range for the maximum removal and decrease the necessary contact time for 50% removal of As(V).
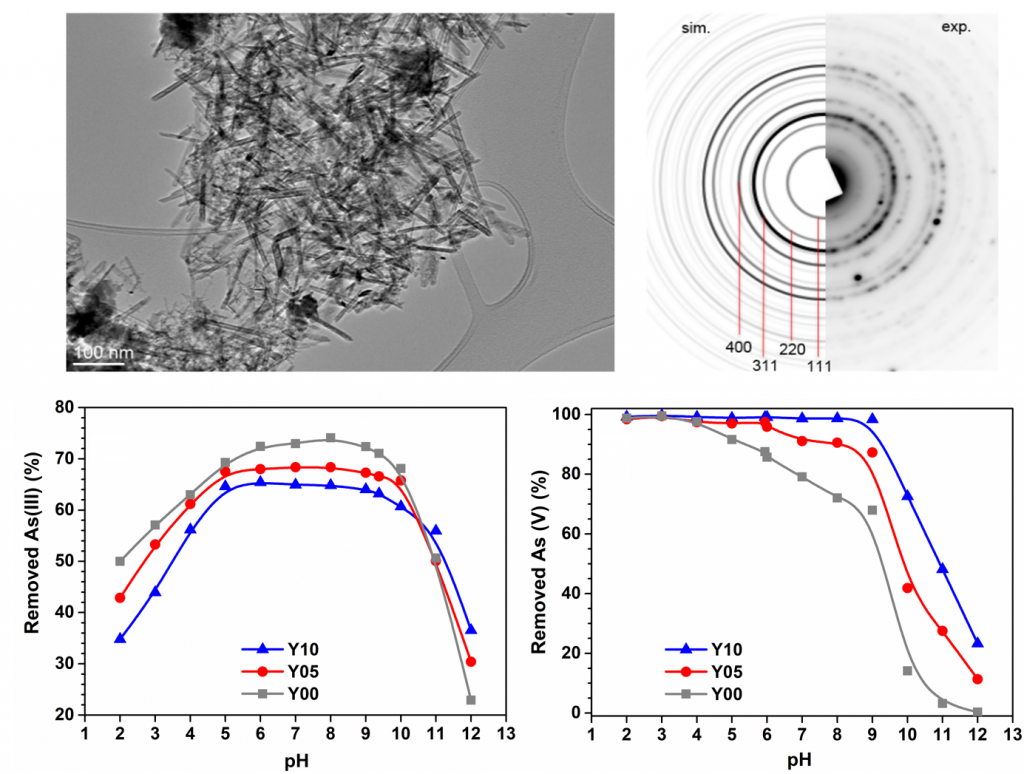
(B. Dojcinovic, et al., Differently shaped nanocrystalline (Fe,Y)3O4 and its adsorption efficiency toward inorganic arsenic species, Nanotechnology 30 (2019) 475702).